Microfluidics Flourish in Jeon's UCI Lab
By Anna Lynn Spitzer
08.01.05
|
-- Research on microfluidic, or nanoliter-scale fluid, devices could one day lead to a cure for breast cancer and spinal cord injuries. By applying the area of microfluidics to biology, researchers working in the lab of Noo Li Jeon, UCI assistant professor of biomedical engineering, are creating devices that could contribute to such a breakthrough.
Jeong Won Park, a biomedical engineering postdoctoral researcher, and Madelyn Luttgen, a third-year biomedical engineering student and SURF-IT research fellow, have developed a microfluidic device that allows scientists to easily replicate tests on the central nervous system.
“Our system can mimic different spinal cord injuries,” Park explains. “After a spinal cord injury, certain myelin-derived proteins prevent axonal regeneration. Many people study these inhibitions through an in vivo model utilizing rats and mice, but with our system, it is much easier to test new drugs and observe the regeneration of axons.”
In vivo models are unreliable for this type of research because conditions differ from test to test. Even minor variations in vivo may trigger vastly different results at the cellular level.
|
||||
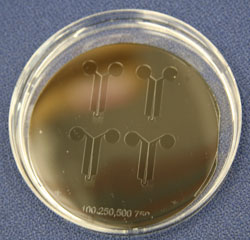
With Park and Luttgen’s microfluidic device, which consists of thousands of parallel, 150-micron-long channels between two vertical channels, scientists can duplicate the exact conditions of each experiment.
“After we put the cell medium and the cells into these wells, they make their way into the microchannels, which are connected like rungs in a ladder,” Luttgen elucidates. “The head of the neuron will grow on one side and the axon on the other side. The axons grow in rows, so it’s easy to manipulate them.”
Bobak Mosadegh, a fourth-year biomedical engineering student and SURF-IT research fellow, is working on a similar microfluidic device developed to study chemotaxis (cell migration in response to external concentration gradients). The device also consists of many microchannels between two vertical channels.
|
By adding epidermal growth factor, a protein that causes migration of cells, to the microchannels and altering the grooves of the channels, researchers are able to generate different concentration gradients and chemical profiles. Certain cells will then migrate across the channels depending on the different concentration gradients.
“We can analyze the cells that pass through to determine which receptors caused them to go through the channels,” Mosadegh says. “Hopefully we can find a relationship between receptors and chemotaxis.”
The lab team has decided to focus the project on two cells types: metastatic breast cancer cells and infection-fighting neutrophils. With new, detailed insight into the migration patterns of these cells in precisely controlled gradients, improved treatment for the prevention of the spread of breast cancer cells and the promotion of wound-healing cells can be developed.
“It’s the basic science of why things happen, which is why we’re able to apply it,” Mosadegh adds.
Both groups have begun experiments; Mosadegh is studying the migration of breast cancer cells and Luttgen is testing the effect of Nogo, a growth-cone inhibitor. Mastering the capabilities of microfluidic devices will open many doors to future research.
“There’s a lot of potential in the field,” Jeon says. “We work on coming up with new devices to look at things that nobody could even dream of looking at before.”